Goal:
To develop genetics-related techniques, especially those essential for maintenance and supply of laboratory mice and stem cell lines at a high quality in Bioresource Center (BRC).
Activities:
- Development of mouse somatic nuclear transfer techniques
- Development of microinsemination techniques
- Development of reliable cryopreservation techniques for mouse embryos or gametes
- Development of new stem cell lines
Specific Aims
1. Development of mouse somatic nuclear transfer techniques
We found that normalization of the Xist expression by gene knockout in cloned mouse embryos remarkably increased the cloning efficiency (Figs 1 and 2).
To seek for a more conventional strategy, we have started RNA interference experiments using cloned mouse embryos. We also found that X-linked genes at A7.2 and F3 were downregulated in cloned blastocysts in an Xist-independent manner. We analyzed these regions for a repressive histone modification, H3K9me2, by ChIP-on-chip using donor cumulus cells.
The results showed that this regions, but not others, were highly enriched with H3K9me2, suggesting that this histone modification may be resistant to reprogramming by nuclear transfer.
Inoue K, Kohda T, Sugimoto M, Sado T, Ogonuki N, Matoba S, Shiura H, Ikeda R, Mochida K, Fujii T, Sawai K, Otte AP, Tian XC, Yang X, Ishino F, Abe K, Ogura A. Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science 330: 496-499, 2010.
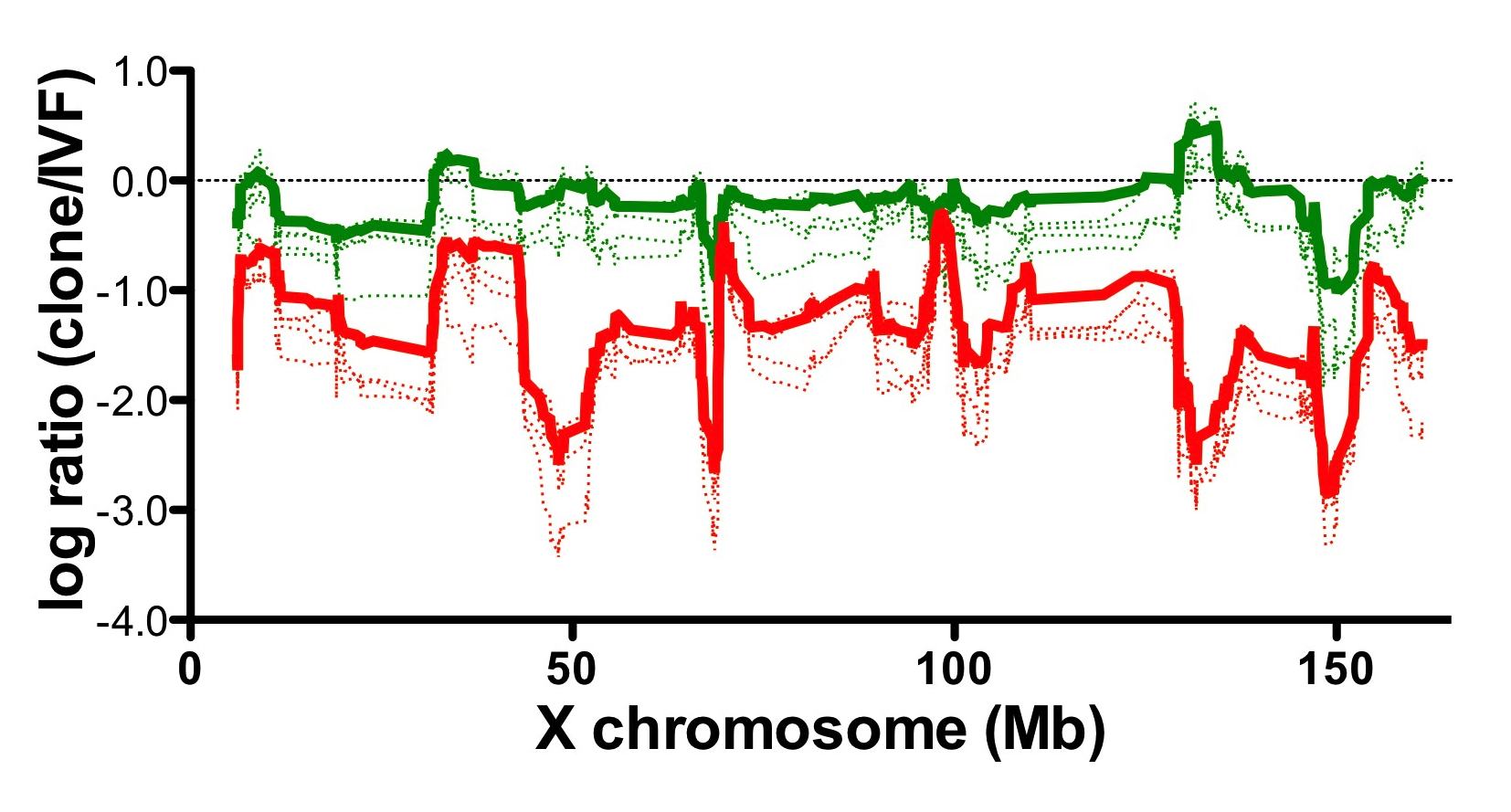
Fig1. Relative gene expression levels of wild type somatic cells and Xist knockout cells cloned embryos.
Gene expression level of somatic cells cloned embryos (red) was downregulated compared with in vitro fertilized control embryos (0 in the graph).
Aberrant gene expression pattern of cloned embryos was improved by using Xist knockout cells as nuclear donors (green).
![]()
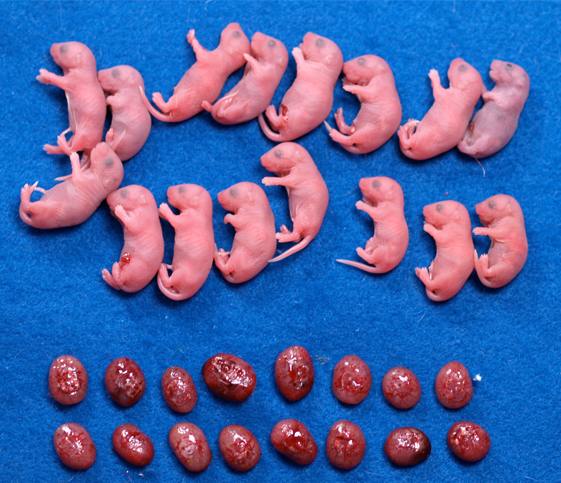
Fig2. Mice cloned from wild type (left) and Xist knockout (right) donor cells.
2. Development of microinsemination techniques
1) The effect on intracytoplasmic sperm injection outcome of genotype, male germ cell stage and freeze-thawing in mice
Mouse ICSI has been applied to a broad range of experiments in reproductive biology using mouse models. To define the factors that affect the efficiency of mouse ICSI, we undertook experiments involving five strains and three male germ-cell types with or without freezing treatment.
Developmental parameters were analyzed by three-way ANOVA. The statistical analysis allowed us to accumulate important information on mouse ICSI. Genotype affected the oocyte survival, cleavage and birth rate, whereas freeze-thawing had no effects on any of the parameters. There were significant genotype/cell type interactions for oocyte survival and cleavage, indicating that they were determined by a combination of strain and germ cell maturity.
Ogonuki N, Mori M, Shinmen A, Inoue K, Mochida K, Ohta A, Ogura A. The effect on intracytoplasmic sperm injection outcome of genotype, male germ cell stage and freeze-thawing in mice. PLoS ONE, 5(6): e11062. doi:10.1371/journal.pone.0011062
2) Birth of normal following round spermatid injection without artificial oocyte activation
For fertilization using round spermatid injection (ROSI) in mice, oocytes need to be artificially preactivated because of the lack of oocyte-activating capacity in round spermatids of this species. However, when round spermatids were frozen-thawed before microinjection, 11-71% of injected oocytes developed into 2-cell embryos without any artificial activation. After being transferred into recipient females, 5-27% of these embryos reached term.
At least some of the injected oocytes showed intracellular Ca2+ oscillations, which normally occur after fertilization by mature spermatozoa. Thus, these round spermatids could transmit a sperm-borne oocyte-activating factor, which might have been released from spermatozoa and elongated spermatids in the same suspension by freezing and thawing.
This possibility was further supported by activation of intact oocytes following transplantation of the pronuclei from ROSI-generated embryos. Thus, one-step ROSI can be achieved in mice simply by injecting frozen-thawed round spermatids into intact oocytes. Clearly, there is a need for careful interpretation of microinjection experiments when assessing the oocyte-activating capacity of spermatogenic cells, especially when they are derived from frozen-thawed stocks.
Ogonuki N, Inoue K, Ogura A. Birth of normal mice following round spermatid injection without artificial oocyte activation. J Reprod Dev (in press)
3) Generation of Functional Oocytes and Spermatids from Primordial Germ Cells
Mammalian germ cells first emerge from epiblast cells in early postimplantation embryos as primordial germ cells (PGCs).
After specification into germ cells, PGCs undergo dynamic genetic and epigenetic changes that are requisites for the formation of functional gametes from PGCs and the generation of new life in the next generation.
Based on the recent development of cell/tissue culture systems and the accumulating knowledge of germ cell biology, many attempts have been made to culture germ cells in vitro by mimicking their development in vivo.
However, the complexness of the process of germ cell development has hampered the induction of immature germ cells into functional gametes in culture conditions.
Here we examined the feasibility of ectopic transplantation of PGCs into adult animals as a means of obtaining functional gametes.
PGCs were isolated from male and female mouse gonads at 12.5 days post coitum (dpc) and transplanted under the kidney capsule of adult mice, together with gonadal somatic cells.
The transplanted cells constructed testis-like and ovary-like tissues, respectively, under the kidney capsule within 4 wk.
Normal-looking round spermatids and fully grown germinal vesicle (GV) oocytes developed within these tissues. The injection of these round spermatids directly into mature in vivo-derived oocytes led to the birth at term of normal pups. PGC-derived GV oocytes were isolated, induced to mature in vitro,
and injected with normal spermatozoa. The injected oocytes were successfully fertilized and developed into normal pups.
These findings demonstrate the remarkable flexibility of PGC development, which can proceed up to functional gametes under a spatially and temporally non-innate condition.
This transplantation system may provide a unique technical basis for the induction of the development of early germ cells of exogenous origins, such as those from embryonic stem cells.
Matoba S, Ogura A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult animals. Biol. Reprod. 84: 631-838, 2011
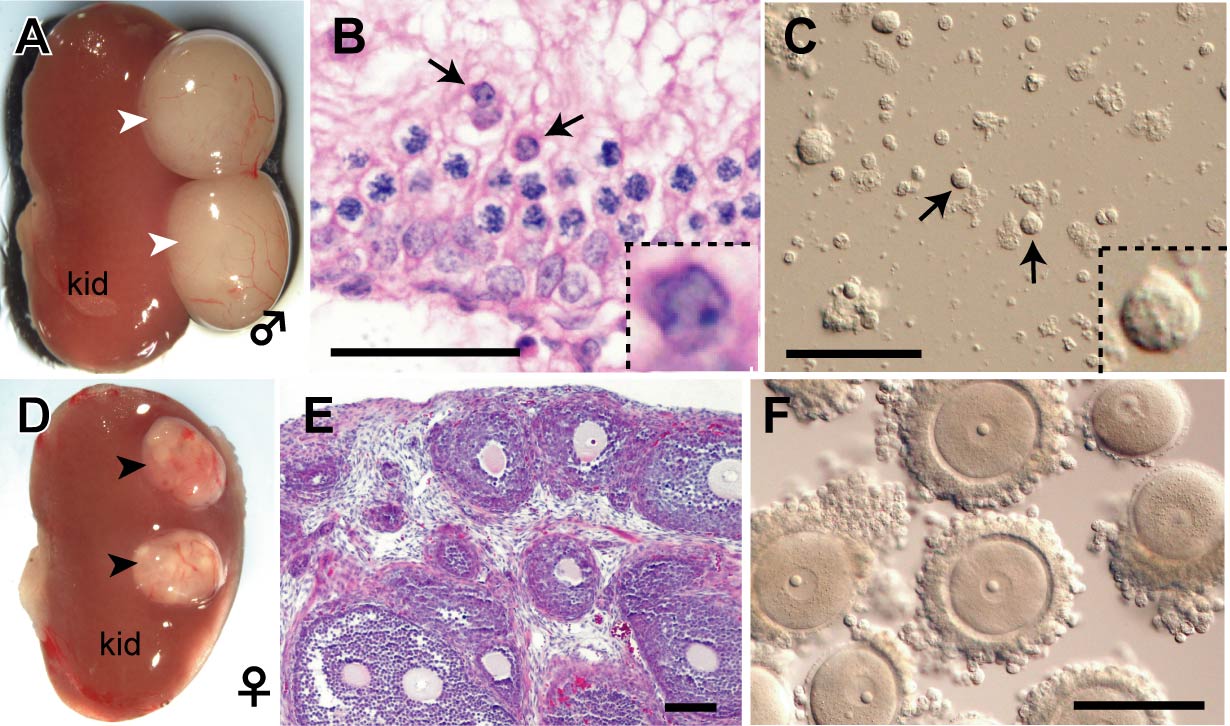
Fig. 3. PGC-derived spermatids and oocytes observed at 4 weeks after transplantation under the kidney capsule.
A-C: Male. D-F: Female. PGCs were transplanted under the kidney capsule together with sex-matched gonadal somatic cells. Male and female grafts formed testis-like (white arrowheads) and ovary-like (black arrowheads) tissues, respectively, depending on the sex of transplanted gonadal somatic cells. In the testis-like tissues, round spermatids (arrows) were formed within the seminiferous tubule. In female, many follicles containing fully grown GV oocytes developed synchronously.
3. Development of reliable cryopreservation techniques for mouse embryos/gametes
1) Development of a novel high osmolality vitrification (HOV) method for cryopreserving mouse embryos
Cryopreservation of mouse embryos uses two major strategies: slow freezing and vitrification. We have developed a novel vitrification method (Fig. 4) with several advantages.
1) The cooling process is simple and quick.
2) The warming process can be performed slowly, as in the slow freezing method.
3) Vitrification can be done at room temperature and does not need a programmable cell freezer.
4) Mouse embryos could survive even at –80 °C for at least 2 months and be transported internationally when packed on dry ice.
5) The 2-cell embryos of major inbred strains are preserved stably with high survivability (93–100%) and a good ability to develop into offspring (33–82%). 6) This method is expected to be applicable for other embryo developmental stages, species and cell types.
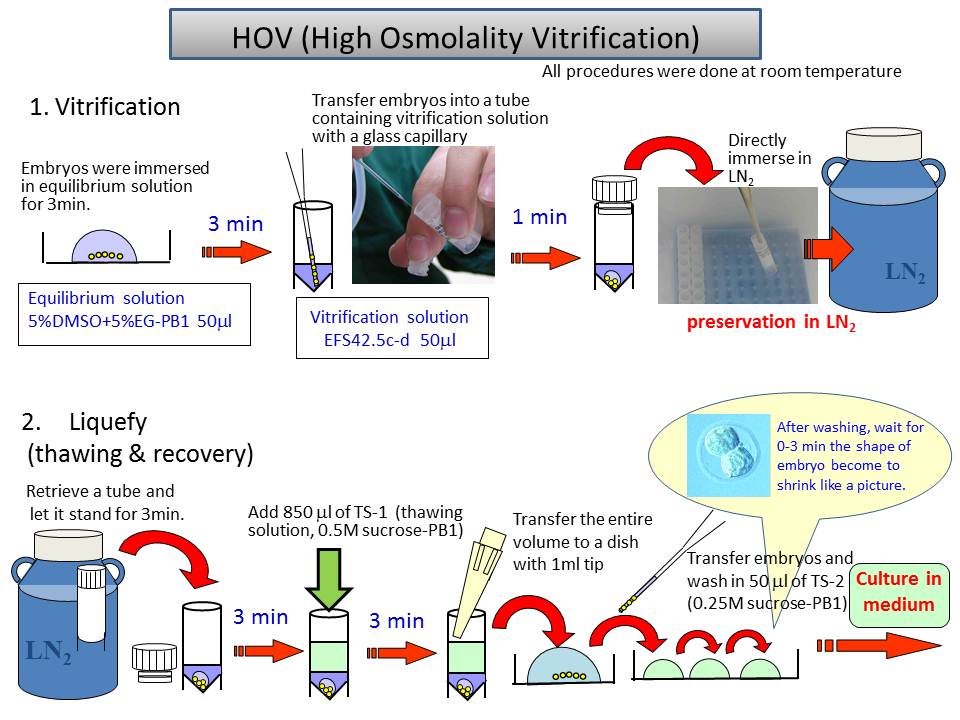
Fig.4 Schema of cryopreservation procedure by HOV method
2) Development of a simple method for freezing mouse spermatozoa in cryotubes
Historically, plastic straws have been preferred as containers for frozen mammalian spermatozoa because this approach gives a high survival rate after thawing. We have developed a new protocol for mouse sperm cryopreservation using cryotubes.
Epididymal sperm were suspended in freezing solution (18% raffinose and 3% skim milk in distilled water) and placed in 10 ml aliquots in cryotubes (no. 366656, Nalge Nunc). The cryotubes were immersed 1 cm below the surface of liquid nitrogen (–196 °C) and then thawed at 50 °C. The fertilization rates using spermatozoa frozen–thawed using this method were 63.1% in ICR and 28.2% in B6 strains of mice.
The latter rate was increased to 69.3% by adding reduced glutathione (GSH: final concentration 1 mM) to the fertilization medium.
Hasegawa A, Yonezawa K, Ohta A, Mochida K, Ogura A. Optimization of a protocol for cryopreservation of mouse spermatozoa using cryotubes. J Reprod Dev. (in press)
3) Development of in vitro fertilization with cryopreserved spermatozoa in major inbred mouse strains
In vitro fertilization was performed with mouse spermatozoa thawed after being cryopreserved in plastic straws.
HTF medium was used for preincubation, supplemented with 0.01% (w/v) PVA and 0.4 mM methyl-b-cyclodextrin instead of BSA. The insemination medium was supplemented with 1.0 mM GSH. Fertilization rates of better than 75% were obtained in most inbred strains except for 129/SvJ and C3H/HeN(Fig. 5).
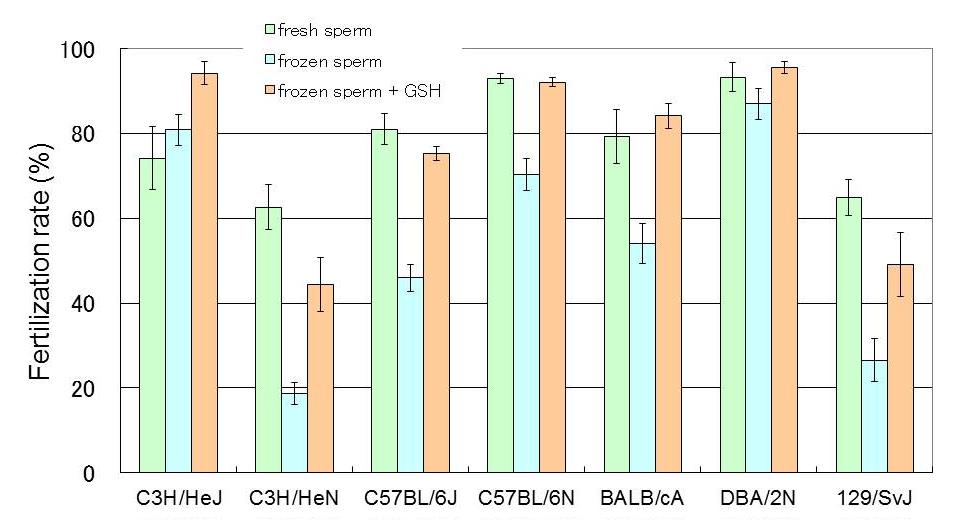
Fig.5 Results of IVF with cryopreserved sperm in several inbred strains
4) Development of an efficient method to produce offspring by improvements in superovulation and embryo transfer in wild-derived strains of mice
Wild-derived mouse strains have an invaluable role in genetic studies based on interstrain polymorphisms. However, most strains show poor reproductive performance in the laboratory.
We used the MSM/Ms strain for superovulation, and injected inhibin antiserum instead of eCG, with hCG given 48 h later. As a result, about 3–4 times more oocytes were ovulated than with conventional eCG–hCG treatment. We then generated embryos by IVF.
However, most such embryos transferred into the oviducts of recipient mice failed to develop to term. Ultimately, we succeeded in improving the survivability of these MSM/Ms embryos by combining treatment of the recipient females with an immunosuppressant (cyclosporine A) and by co-transferring embryos from laboratory strains.
4. Development of new stem cell lines
We have developed effective methods to pluripotent stem cells from several mouse strains and rabbits, as experimental models for translational research and the conservation of genetic resources.
1) Mouse Embryonic Stem Cells
To generate ”high quality stem cells” is necessary not only for producing genetically engineered mouse (animals) but also for developing human regenerative medicine. To these ends, we have used the 2i media which improve the potency of preexisting C57BL/6 ES cells to produce high-contribution and germline competent chimeric mice (Fig.6- A). We have obtained high quality C57BL/6 ES cells as a gold standard of pluripotent stem cells in the other strains or animal species.
2) Rabbit Pluripotent Stem Cells
Although pluripotent stem cell lines derived from mice and primates are used extensively, the development of such lines from other mammals is extremely difficult because of their rapid decline in proliferation potential and pluripotency after several passages.
Laboratory rabbits have long been used in biomedical research. Recently, they have been used as experimental models for human diseases. Rabbit ES cells would be invaluable for the study of human diseases using gene-targeted technology and for testing of stem cell therapies for human applications.
We have successfully developed an efficient and reproducible technique for the establishment of rabbit ES cells.
Moreover, we have showed that the major important characters of rabbit ES cells are common with human ES cells.
We have successfully reprogrammed adult rabbit somatic cells to form colonies of fully pluripotent cells that are highly similar to rabbit ES cells.
These induced pluripotent stem (iPS) cells of rabbits are very similar to their ES cell counterparts (Fig. 6-B). On the other hand, although the global gene expression profiles became closer to those of the rabbit
ES cells with cell passage proceeded, a slight but rigid difference between the two types of rabbit pluripotent stem cells was revealed. Recently, we are establishing the systems for assessing the quality of the rabbit ES/iPS cell lines using the in vitro differentiation ability as an indicator.
Next our objective is to improve rabbit ES/iPS cell lines to appropriate quality for applying translational research model and for producing genetically engineered rabbits.
Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, Ogura A. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Biol Chem, 285: 31362-31369, 2010.
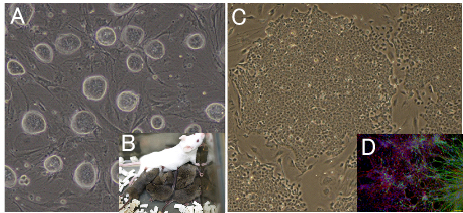
Fig6. Development of new stem cell lines
(A) Mouse ES cell colonies after “2i” treatment. Colonies are closely packed and have
(B) 2i treated ES cells obtained the ability to transmit to germline.
(C) Rabbit iPS cell colonies, which resembles rabbit ES cell colonies.
(D) It can be differentiated to a neural lineage. Neural differentiation is used for assessing the quality of the rabbit ES cells and iPS cells.
Green: Tuj1 for neural tube, Red: GFAP for astrocyte.
